Clinical Research Associate I Salary in the United States
Clinical Research Associate I Salary
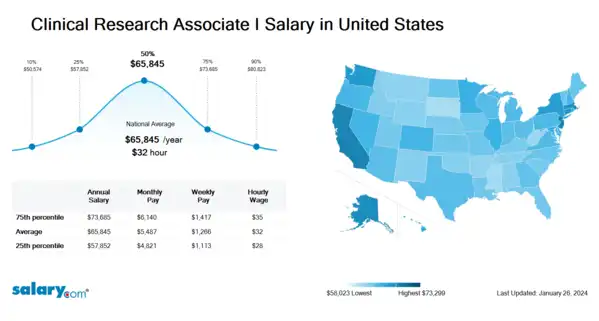
How much does a Clinical Research Associate I make in the United States? The average Clinical Research Associate I salary in the United States is $66,166 as of March 26, 2024, but the range typically falls between $58,136 and $74,049. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, the number of years you have spent in your profession. With more online, real-time compensation data than any other website, Salary.com helps you determine your exact pay target.
| Percentile | Salary | Location | Last Updated |
| 10th Percentile Clinical Research Associate I Salary | $50,824 | US | March 26, 2024 |
| 25th Percentile Clinical Research Associate I Salary | $58,136 | US | March 26, 2024 |
| 50th Percentile Clinical Research Associate I Salary | $66,166 | US | March 26, 2024 |
| 75th Percentile Clinical Research Associate I Salary | $74,049 | US | March 26, 2024 |
| 90th Percentile Clinical Research Associate I Salary | $81,225 | US | March 26, 2024 |
METCUT RESEARCH ASSOC - Cincinnati, OH
Budget Analyst Associate - REMOTE
Planet Pharma - Cleveland, OH
Associate Counsel, P&C Insurance Fronting Contract Drafting/Negotiating (Remote, East Coast prefe...
Response Companies - Cleveland, OH
Clinical Research Coordinator II - RI Nathan
Nationwide Children's Hospital - Columbus, OH
- View Hourly Wages
-
Select State
-
Select City
-
Choose Similar Job
-
Pick Related Category
- View Cost of Living in Major Cities
What skills does a Clinical Research Associate I need?
Each competency has five to ten behavioral assertions that can be observed, each with a corresponding performance level (from one to five) that is required for a particular job.
Integrity: Is about having strong principles and values, which you demonstrate through your conduct in the work environment. A common integrity definition states that people with integrity do the right thing even when nobody is watching.
Clinical Trial Management System: A clinical trial management system (CTMS) is a software system used to manage clinical trials in clinical research. This CTMS will serve as a single, centralized, web-based enterprise resource to support clinical research studies conducted within or across the three institutions.
Clinical Operations: Clinical Operations refer to the activities that support the clinical trial process from start-up to close out. Ensures there is proper planning, appropriate conduct through the process, safety of patients and use of quality data.
Job Description for Clinical Research Associate I
Clinical Research Associate I participates in the design, administration and monitoring of clinical trials. Analyzes and evaluates clinical data gathered during research. Being a Clinical Research Associate I ensures compliance with protocol and overall clinical objectives. Knowledge of FDA regulatory requirements is required. Additionally, Clinical Research Associate I may require ACRP or SOCRA Clinical Research Professional exam completion. Requires a bachelor's degree in Science or its equivalent. Typically reports to a supervisor or manager. The Clinical Research Associate I work is closely managed. Works on projects/matters of limited complexity in a support role. To be a Clinical Research Associate I typically requires 0-2 years of related experience. (Copyright 2024 Salary.com)... View full job description
See user submitted job responsibilities for Clinical Research Associate I.
Search Job Openings
Salary.com job board provides millions of Clinical Research Associate I information for you to search for. Click on search button below to see Clinical Research Associate I job openings or enter a new job title here.
Career Path for Clinical Research Associate I
A career path is a sequence of jobs that leads to your short- and long-term career goals. Some follow a linear career path within one field, while others change fields periodically to achieve career or personal goals.
For Clinical Research Associate I, the upper level is Clinical Research Associate II and then progresses to Clinical Research Manager.
What does a Clinical Research Associate I do?
Are you an HR manager or compensation specialist?
Salary.com's CompAnalyst platform offers:
- Detailed skills and competency reports for specific positions
- Job and employee pricing reports
- Compensation data tools, salary structures, surveys and benchmarks.

Clinical Research Associate I Pay Difference by Location
Clinical Research Associate I salary varies from city to city. Compared with national average salary of Clinical Research Associate I, the highest Clinical Research Associate I salary is in San Francisco, CA, where the Clinical Research Associate I salary is 25.0% above. The lowest Clinical Research Associate I salary is in Miami, FL, where the Clinical Research Associate I salary is 3.5% lower than national average salary.
| City, State | Compared to national average |
|---|---|
| City, State San Francisco, CA |
Compared to national average
|
| City, State Washington, DC |
Compared to national average
|
| City, State Miami, FL |
Compared to national average
|
| City, State Chicago, IL |
Compared to national average
|
| City, State Boston, MA |
Compared to national average
|
| City, State New York, NY |
Compared to national average
|
| City, State Dallas, TX |
Compared to national average
|
Similar Jobs to Clinical Research Associate I

| Job Title | Experience | EDUCATION | Salary Compared to This Job |
|---|---|---|---|
| Job Title Clinical Research Associate II | Experience 2 - 4 | EducationMasters | Salary Compared to This Job |
| Job Title Clinical Research Manager | Experience 5 + | EducationMasters | Salary Compared to This Job |
| Job Title Fraud Prevention Associate I | Experience 1 - 3 | EducationHigh School | Salary Compared to This Job |
| Job Title Research and Development Associate I | Experience 0 - 2 | EducationBachelors | Salary Compared to This Job |
| Job Title Research and Development Associate II | Experience 2 - 4 | EducationBachelors | Salary Compared to This Job |
Level of Education for Clinical Research Associate I
Jobs with different levels of education may pay very differently. Check the Clinical Research Associate I salary of your education level.
Clinical Research Associate I Salary by Global Country
Clinical Research Associate I salary varies from country to country. There are several factors that mainly impact the Clinical Research Associate I salary, including cost of living, economic conditions, market rates and legal differences. Click below to Clinical Research Associate I salary of the other country.
Clinical Research Associate I Salary by State
Geographic variations impact Clinical Research Associate I salary levels, due to various factors, such as cost of living, industries, market demand and company budgets. Click below to see pay differences between states.
Browse All Pharmaceuticals Jobs by Salary Level
Browse Related Job Categories With Clinical Research Associate I
A job category is a classification or grouping of job positions that share similar characteristics, functions, or industries. Clinical Research Associate I salary varies from category to category. Click below to see Clinical Research Associate I salary in different categories.
Take just three simple steps below to generate your own personalized salary report
Understand the total compensation opportunity for a Clinical Research Associate I, base salary plus other pay elements
Average Base Salary
Core compensation
Average Total Cash Compensation
Includes base and annual incentives
View the Cost of Living in Major Cities
Skills associated with Clinical Research Associate I: Research and Development, Clinical Research, Clinical Data Management, Medical Writing ...More
Recently searched related titles: Graduate Research Assistant, Psychology Research Assistant, Clinical Science Liaison
Recently searched companies with related titles : Covance, Inc Clinical Research Associate
Recently searched related titles: Field Clinical Representative, Post Doctoral Research Associate, Senior Clinical Data Associate
Jobs with a similar salary range to Clinical Research Associate I : Clinical Research Monitor, Clinical Training Specialist, Clinical Trial Administrator, Lead Clinical Research Associate, Clinical Associate, Clinical Associate I
Salary estimation for Clinical Research Associate I at companies like : Rtvf, Columbia Radiology MRI Center at Neurological Institute, River Manufacturing LLC
Jobs with a similar salary range to Clinical Research Associate I : Research Clinician, In House Clinical Research Associate, Medical Research Assistant